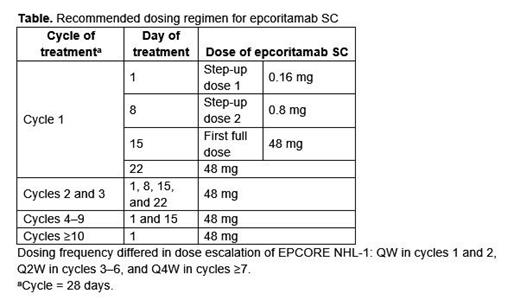
Background: Epcoritamab SC, a CD3xCD20 bispecific antibody developed using the DuoBody ® platform, engages T cells to target CD20-expressing B cells. Epcoritamab was granted approval by the US FDA in May 2023 for the treatment of adults with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL), not otherwise specified, including DLBCL arising from indolent lymphoma, and high-grade B-cell lymphoma after ≥2 lines of systemic therapy. The recommended dosing regimen for epcoritamab is presented in the Table.
Methods: A wide range of epcoritamab doses was explored in patients with R/R B-cell non-Hodgkin lymphoma, including priming doses (0.004-0.16 mg), intermediate doses (0.25-1.6 mg), and full doses (0.0128-60 mg). To identify the optimal dosing regimen for epcoritamab, comprehensive analyses of clinical safety, efficacy, pharmacokinetic (PK), and pharmacodynamic (PD) data (including PK/PD modeling and exposure-efficacy and exposure-safety analyses) were conducted using data from patients with R/R large B-cell lymphoma (LBCL) in the phase 1/2 EPCORE™ NHL-1 (NCT03625037) and EPCORE NHL-3 (NCT04542824) trials with a clinical data cutoff of Jan 31, 2022. Logistic regression analyses were performed to investigate the relationship between epcoritamab exposure and efficacy and safety across the entire range of studied doses.
Results: Treatment with the approved dosing regimen led to a high overall response rate (ORR; 63%) and complete response (CR) rate (39%) and durable responses in this highly refractory patient population (median duration of response, 12.0 mo [95% CI, 6.6-not reached]; Thieblemont et al, JCO 2023). Overall, the safety profile of epcoritamab with the recommended dosing regimen is manageable. Higher exposure was associated with higher ORR ( P<0.0005 for the EPCORE NHL-1 LBCL population; n=192, 116 [60%] with response) and CR rate ( P<0.0005 for the EPCORE NHL-1 LBCL population; n=192, 74 [39%] with CR). Additionally, higher exposure improved progression-free survival ( P<0.0005 for the EPCORE NHL-1 LBCL population; lower 50%, n=97; upper 50%, n=98) and overall survival ( P=0.003 for the EPCORE NHL-1 LBCL population; lower 50%, n=97; upper 50%, n=98). The duration of response data exhibited a similar numerical trend ( P=0.278 for the EPCORE NHL-1 LBCL population; lower 50%, n=58; upper 50%, n=58). The pooled analysis of EPCORE NHL-1 and EPCORE NHL-3 LBCL populations showed similar trends. Importantly, a potential plateau of efficacy was observed at 48 mg or above. Furthermore, the majority of initial responses (94%) were achieved within the first 3 cycles (QW dosing interval), with a median time to response of 1.4 mo and a median time to CR of 2.7 mo in the expansion portion of EPCORE NHL-1 (Thieblemont et al, JCO 2023). Most responders maintained or improved their responses during the subsequent Q2W and Q4W dosing schedules, independent of exposure (C trough) during these periods. The step-up doses (0.16/0.8 mg) were clinically active and helped mitigate the risk of CRS at the subsequent therapeutic full (48-mg) doses. Complete B-cell depletion was observed as early as following the first priming dose. Very few first CRS events occurred after the second full dose or subsequent doses in patients who had not previously experienced CRS. The exposure-safety analysis did not identify any safety concerns with the approved dosing regimen. The rate of AEs, including grade ≥3 treatment-emergent AEs (TEAEs), serious TEAEs, grade ≥3 neutropenia, grade ≥3 infections, TEAEs leading to dose delay, TEAEs leading to treatment discontinuation, any-grade CRS, grade ≥2 CRS, CRS requiring tocilizumab, ICANS, and clinical tumor lysis syndrome, did not increase with increasing epcoritamab exposure.
Conclusions: Our analyses informed the optimal dosing regimen for epcoritamab. Clinically active step-up doses (0.16/0.8 mg) demonstrated antitumor activity and mitigated the risk of CRS at subsequent therapeutic full doses. The recommended full dose (48 mg) provided deep and durable responses and a manageable safety profile in patients with LBCL. Lower full doses resulted in compromised efficacy without improvement of the benefit-risk profile, whereas increasing the full dose is not expected to lead to significantly higher efficacy. The recommended dosing intervals for epcoritamab treatment not only induced rapid responses but also maintained or improved responses during treatment.
Disclosures
Li:Genmab: Current Employment. Gibiansky:Genmab: Consultancy; QuantPharm LLC: Current Employment; F. Hoffmann-La Roche Ltd: Consultancy. Parikh:AbbVie: Current Employment, Current equity holder in publicly-traded company. Chiu:Genmab: Current Employment. Sacchi:Genmab: Current Employment. Feng:Genmab: Current Employment. Gupta:Genmab: Current Employment. Xu:Genmab: Current Employment.